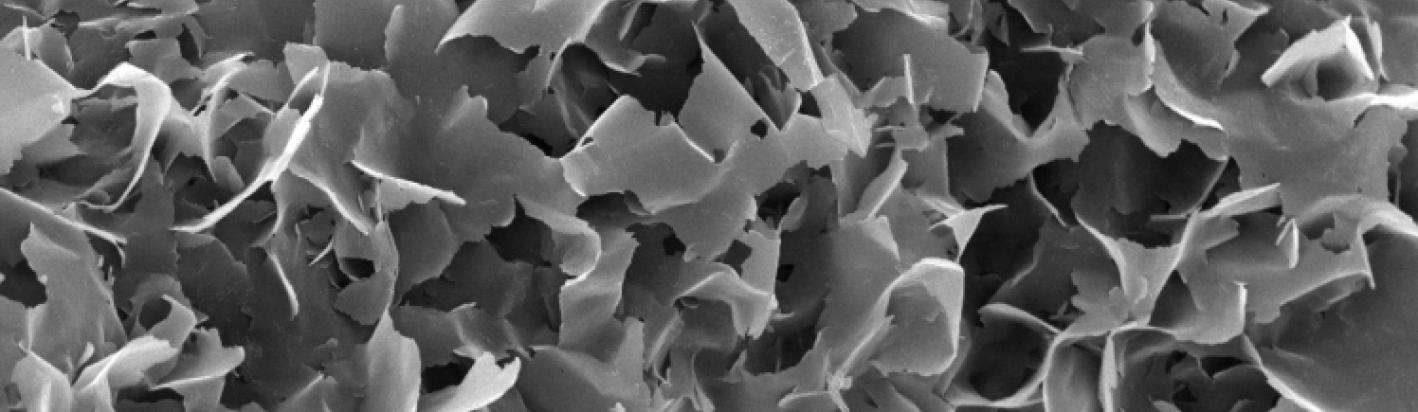
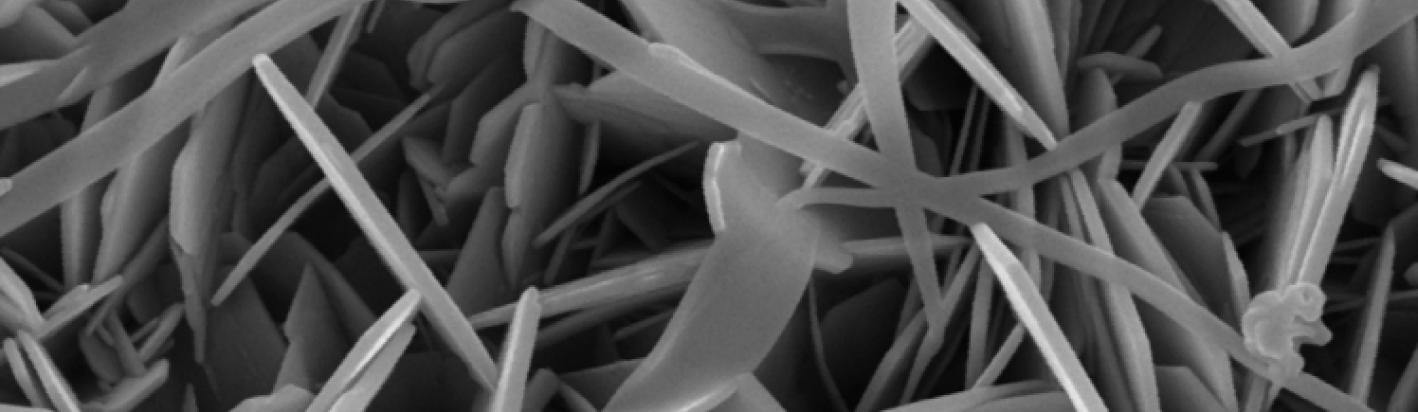
With increasing regulation surrounding crystalline silica it can be important to determine is the silica phase that occurs in some bentonites, or other materials, is opaline silica, such as opal-C, or opal-CT, or crystalline silica in the form of cristobalite.
Distinction between opal and cristobalite can be confirmed by the NaOH method described by Hillier and Lumsdon (2008), (Clay Minerals V43, pp 477-486). (See Publications for a copy.)
The sample is oven dried overnight at 105ºC and allowed to re-equilibrate with the ambient laboratory atmosphere for 2 hours to reach stable moisture content. Five grams of sample are then weighed-out accurately using a four-place calibrated balance and transferred to a 1 litre Pyrex glass beaker containing 500 ml of boiling 0.5 M NaOH. The beaker and its contents are covered, and boiled and stirred continuously with a magnetic stirrer for precisely 10 minutes. They are then cooled rapidly by immersion in cold water, allowed to clear by flocculation, and the sample recovered by removing the liquid using a combination of suction decantation and centrifugation. The recovered clay is again oven-dried, allowed to re-equilibrate and an accurate weight recorded. The recovered clays are then prepared for XRPD analysis to verify the components dissolved by comparison to the XRPD pattern of the original sample. If the silica phase is dissolved the phase is confirmed as opal, since cristobalite is not dissolved by this treatment. The weight loss should also correspond to the amount of silica phase determined by XRPD full pattern fitting.
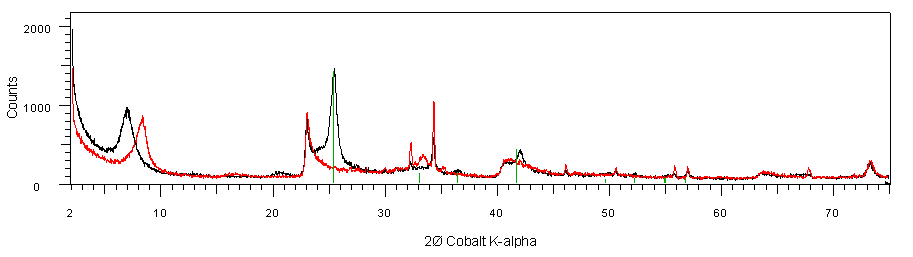
Bentonite before (black) and after NaOH dissolution (red), showing disappearance of peaks (green) due to silica phase which is thus confirmed as opaline silica. The shift in spacing of the smectite component is due to saturation with Na+ following the NaOH treatment.